For decades, Dr. Christopher Gobler has been producing groundbreaking studies revealing the relationship between sewage pollution, harmful algal blooms, ocean acidification, and marine ecosystems. He is a professor at the School of Marine and Atmospheric Sciences at Stony Brook University, Long Island, New York, and editor-in-chief of the peer-reviewed journal Harmful Algae. He’s also a founder of the Center for Clean Water Technology where he and collaborators have developed simple, easy-to-build household onsite septic systems that could solve many of the problems he’s been documenting for three decades.

Dr. Christopher Gobler. Source: Dr. Christopher Gobler
You’ve been researching the link between nitrogen and harmful algal blooms for decades. Contrary to conventional wisdom, which assumes fertilizer is the major source of nitrogen in coastal ecosystems, your research has pinpointed wastewater as a major culprit?
Yes, in some early studies,1 we identified the strong role that wastewater and sewage can play in promoting harmful algal blooms. We collected cells from one very large and dense toxic bloom in the New York region and used an approach that allowed us to understand where the nitrogen in the cells was coming from. Essentially you can get two answers: that it’s coming from fertilizer or it’s coming from wastewater. Or you can get a mixture of the two. The signal on these cells was very strongly wastewater—from septic tanks, septic systems, and sewage treatment plants. More than a dozen modeling studies on Long Island and the Northeast US have concluded that wastewater is the biggest source of nitrogen from land to sea. That was really eye-opening.
More than a dozen modeling studies on Long Island and the Northeast US have concluded that wastewater is the biggest source of nitrogen from land to sea.
How can you tell whether nitrogen is from a septic system or whether it’s from fertilizer?
Essentially you’re looking at the relative abundance of nitrogen atoms that have a molecular weight of 14 and a molecular weight of 15. That’s what they call the isotopic composition of the nitrogen. The process of making fertilizer makes the nitrogen depleted in Nitrogen-15 and more rich Nitrogen-14, so it’s lighter. Conversely, wastewater is heavier in Nitrogen-15 and lighter in Nitrogen-14. That’s why you can very clearly distinguish those two. When fertilizer is made, you’re taking nitrogen in the atmosphere and ‘fixing’ it into something solid. That chemical reaction favors the lighter nitrogen and therefore it’s lighter. Conversely, when we eat food, it’s easier for our bodies to retain the lighter nitrogen, and we pass the Nitrogen-15, therefore wastewater is rich in Nitrogen-15. Isotopic composition is a useful tool that’s been used for more than 30 years to identify the source of a molecule of nitrogen. Seeing how much of the nitrogen was Nitrogen-15 was the first hint that, ‘Wow, wastewater looks to be really important.’
You’ve found that nitrogen not only causes harmful algal blooms, but also makes them more toxic?
Yes. Some of the compounds that these toxic blooms make, such as saxitoxin, which causes Paralytic Shellfish Poisoning, have a chemical structure that’s rich in nitrogen. We found out that the more nitrogen the blooms have available to them, not only are they growing more, but each individual cell can make and contain more toxin. And, it works both ways. If they’re starved for nitrogen, the bloom will be less toxic. And these toxins can accumulate in shellfish to become a public health threat or can be damaging to aquatic life.
The more nitrogen the blooms have available to them, not only are they growing more, but each individual cell can make and contain more toxin.
You’ve also found that the nitrogen in wastewater contributes to ‘dead zones,’ and dead zones contribute to ocean acidification? How does that chain reaction work?
In most marine ecosystems, particularly coastal ecosystems, nitrogen is the ‘limiting element,’ which means that the more you put in, the more you’re going to fuel algae growth. Eventually, the blooms die off and then all of that carbon-rich organic algae sinks to the bottom. As that algae decays, all of the oxygen in the water gets used up. Just as people eat food, inhale oxygen, and exhale CO2, microbes are doing the same thing in the ocean as they eat that carbon-rich algae. They’re eating and they’re taking in oxygen. This is what creates what’s called hypoxic or low-oxygen ‘dead zones.’ All life outside of some bacteria need oxygen to survive, so animals that can flee, like fish, will exit these dead zone, whereas sessile animals like bivalves may perish.
But another part of the equation is that for every molecule of oxygen the microbes consume, they produce a molecule of CO2. The term ocean acidification is usually used in the context of the combustion of fossil fuels. CO2 levels are rising in the atmosphere, entering our oceans, and that can drive down the pH in certain places. But CO2 has the same effect on ocean chemistry whether it’s coming in from the atmosphere or being generated internally. We run boats with sensors that test and map water pH, and many of our water samples from all over the Northeast show pH levels as low as 7, which is extremely low. The ocean typically has a pH of around 8.1. What we began to realize is that all of these dead zones are also likely to be acidification zones as well. What’s the cause? Many times, it’s the overloading of nitrogen from wastewater. We wrote a paper on this called, Coastal Ocean Acidification, the Other Eutrophication Problem.
Why is acidification bad for ocean health?
All marine life lives within an optimal pH balance. When marine waters acidify, it can endanger any organism, but it is most threatening to animals that make hard parts out of calcium carbonate like corals and shellfish. When the pH drops, make those shells is more difficult. For early life stages, acidification can be lethal.
There is concern that if we combust all the fossil fuels on the planet in a couple of centuries, the total pH of the ocean could get as low as 7.7 or 7.6. But we’re seeing today, in 2020, pH levels far below that. So when it comes to sewage discharge and the overloading of nutrients and acidification, we don’t need to wait for the future. It’s here, now.
All of this can start to feel like an overwhelming problem, but I know you’ve also been working on solutions. What are some of the most promising interventions?
There are plenty of examples of “good news” stories, and it’s clear, taking action leads to improvements. Not taking action leads to degradation. For instance, in New York City, the East River —which is not really a freshwater river but a tidal passage—runs along Manhattan and the Bronx and through Brooklyn and Queens and drains into the Long Island Sound. Along the way, several sewage treatment plants drain into it, and until recently, it had the second- or third-biggest dead zone in the United States. In recognition of that, the EPA issued a mandate that New York City needed to cut the nitrogen discharging into the East River by 60 percent. NYC invested $1 billion to upgrade four treatment plants so that they do a better job of removing nitrogen, and since then the dead zone has progressively shrunk year after year. And, on the south side of Long island a sewage treatment plant had been draining directly into a place called the Great South Bay. After a project to divert the discharge away from the bay, thousands of acres of seagrass meadows regrew in the area where the sewage had previously been discharged. And, in the other end of the bay, where they didn’t control the nitrogen, studies showed they had lost thousands of acres of seagrass beds.
What are the upgrades to treatment plants that allow them to remove nitrogen?
You can add the process of denitrification to the sewage treatment plant. It’s called BNR—biological denitrification—and creates conditions that force bacteria to pull nitrate out of the water. You have to sort of trick them into doing it. The water is aerated and that makes certain bacteria convert ammonium to nitrate. Then they let the water sit. Oxygen levels go to zero and the bacteria take the nitrate and convert it to nitrogen gas (N2), which makes up 80 percent of our atmosphere.
You’ve also set up a lab to develop solutions for places that don’t have sewage treatment plants. What have you come up with?
As I mentioned, in New York City, they invested in upgrading their sewage treatment plants because that’s where the nitrogen is coming from. But in the eastern three quarters of Long Island most of the homes are not connected to sewage treatment plants. In those cases, the majority of nitrogen going from land to sea is from onsite septic systems. The most cost-effective way to deal with those is to have individuals use what we call “innovative and alternative onsite wastewater treatment units.” At the New York State Center for Clean Water Technology, we’ve designed three prototypes, which we call nitrogen-removing biofilters, and they’re all achieving nitrogen effluent that’s on par with sophisticated sewage treatment plants.
Can you describe how these systems that you’ve designed work?
They’re very, very, very simple. They consist of in-the-ground layers of sand and layers of sand mixed with a source of carbon, typically wood chips, and that creates the same setting used at sewage treatment plants. When the water hits the sand, it promotes nitrification—where ammonium (a source of nitrogen in human waste) is converted to nitrate. After that, the water trickles down into the layer with the wood chips, where denitrifying microbes convert the nitrate into nitrogen gas.
What’s next? How close are you to being able to start scaling up and getting people to convert to using these systems?
In Suffolk County, there’s an official process for getting these systems approved. We’re now in stage 2 of 3. The standard for effluent is that it must be, on average, below 19 mg/L of nitrogen. Our systems on average produce effluent between 5 and 10 mg/L, so we’re having no problem making the grade. Now, we’re in the piloting stage. We’re also preparing guidance documents, which will be available for the public. Once those are available, we’ll get approval for installation across Long Island. These systems are what we call non-proprietary, so anyone could install this system. And all you need is some clean sand, wood chips, PVC piping, and the ability to get a backhoe in, to move some earth.
Would this system you’re describing work in a densely populated area where there’s less space?
What I described so far is what we call our ‘first generation’ systems, which require a little bit of a yard, but we have a new system that we’re working on right now that’s even more efficient at removing nitrogen and is able to get it down to below 3mg/L. It’s really just a box, about 10′ x 4′ x 6′, and we think this could be better suited for a densely populated area.
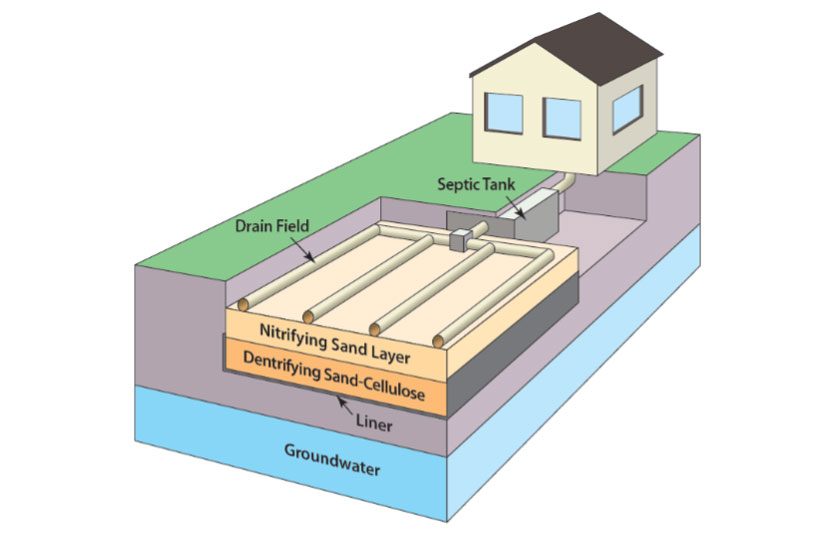
Diagram of nitrogen-removing biofilter prototype. Source: Dr. Christopher Gobler
I’m guessing what a lot of people will want to know is, what’s the cost?
Some of these onsite systems right now are in the vicinity of $20,000. Our goal is $10,000. At the Center we have a “10-10-30” goal: Develop onsite systems that get nitrogen down below 10 mg/L, cost less than $10,000, and last for at least 30 years. The US EPA drinking water standard for nitrate is 10mg/L and emerging epidemiological research suggests even lower levels can promote some types of cancers.2,3 On Long Island, our drinking water comes from groundwater, so we want to protect that resource to the best of our abilities.
And, you’ve got two of the three.
Yes. We’re below the 10mg/L, and we’re confident that the materials we’re using will last more than 30 years. So now it’s about getting the cost down.
Any final thoughts?
Nitrogen from sewage discharge has an overarching effect on ecosystems. The more I study, the more amazed I am. It’s the algae, the harmful algae blooms, the loss of seagrass, the loss of salt marshes, the hypoxia, the acidification, and the loss of fisheries. If you follow the bread crumbs in coastal ecosystems, in many cases, wastewater can be the strongest source of nitrogen, which can then have a strong organizing effect on all other systems.
Or disorganizing, as the case may be.
Yeah. Chaos.
If you follow the bread crumbs in coastal ecosystems, in many cases, wastewater can be the strongest source of nitrogen, which can then have a strong organizing effect on all other systems.
Notes
- Hattenrath, T. K., Anderson, D. M., & Gobler, C. J. (2010). The influence of anthropogenic nitrogen loading and meteorological conditions on the dynamics and toxicity of Alexandrium fundyense blooms in a New York (USA) estuary. Harmful Algae, 9(4), 402-412.
- Barry, K. H., Jones, R. R., Cantor, K. P., Beane Freeman, L. E., Wheeler, D. C., Baris, D., Johnson, A. T., Monawar Hosain, G., Schwenn, M., Zhang, H., Sinha, R., Koutros, S., Karagas, M. R., Silverman, D. T., and Ward, M. H. (2020). Ingested nitrate and nitrite and bladder cancer in northern New England. Epidemiology, 31(1), 136-144.
- Temkin, A., Evans, S., Manidis, T., Campbell, C., and Naidenko, O. V. (2019). Exposure-based assessment and economic valuation of adverse birth outcomes and cancer risk due to nitrate in United States drinking water. Environmental Research, 176.
